MT-Prep™ XL
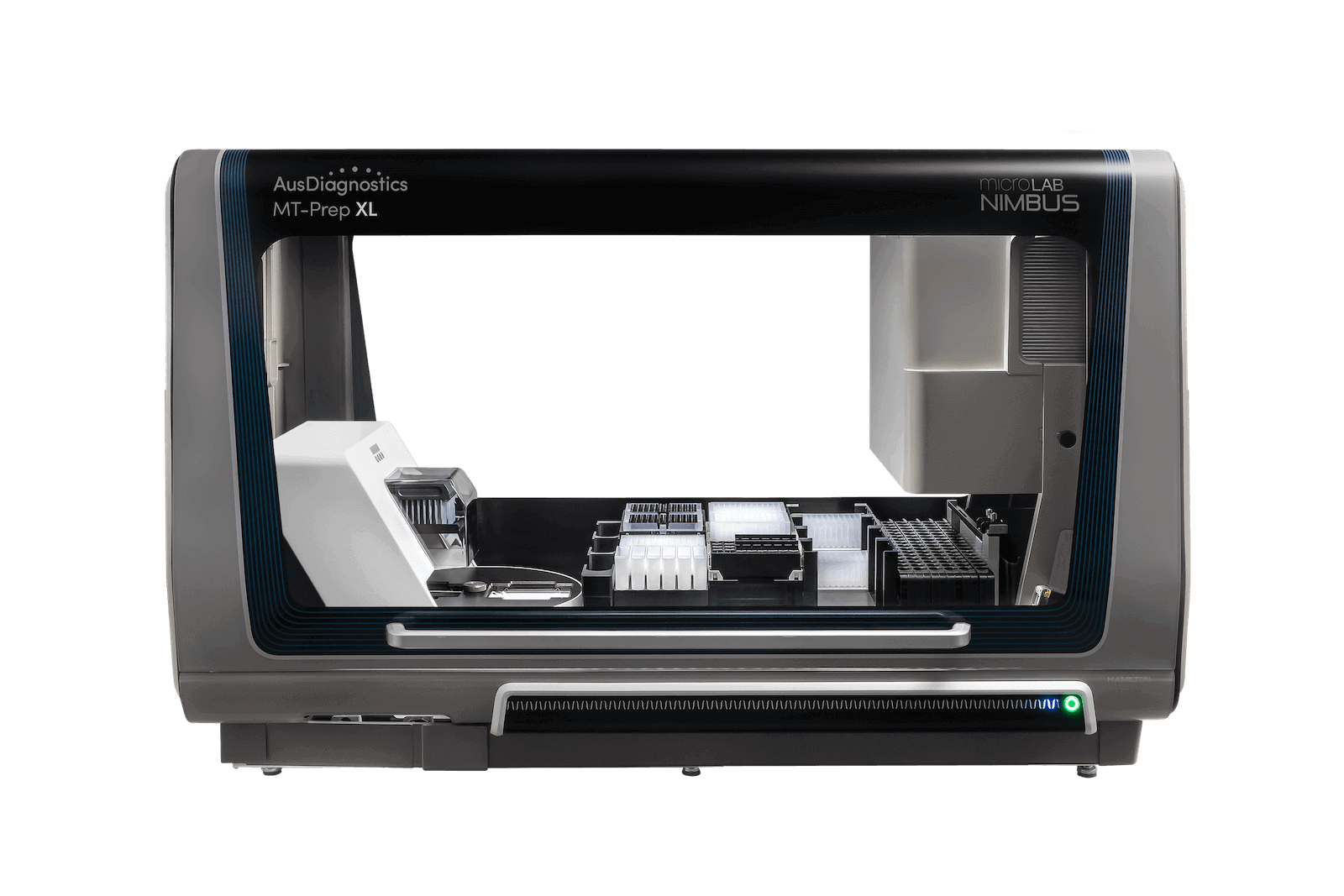
Fully automated sample purification for up to 96 samples in 1 hr 15 mins
MT-Prep™ XL is the synergy between innovative Hamilton robotics with custom-designed Kingfisher Presto technology. This platform enables fully automated high-throughput sample purification.
Total processing time (96 samples):
1 hr 15 mins
Total hands-on time:
10 mins
Features & Benefits
- Universal extraction - process different sample types at the same time
- Minimal 10 minutes hands-on time
- Automatic barcode scanning for end-to-end traceability
- Dual-position turntable for parallel processing
- Use with Puryx® Comprehensive RNA/DNA Extraction Kits
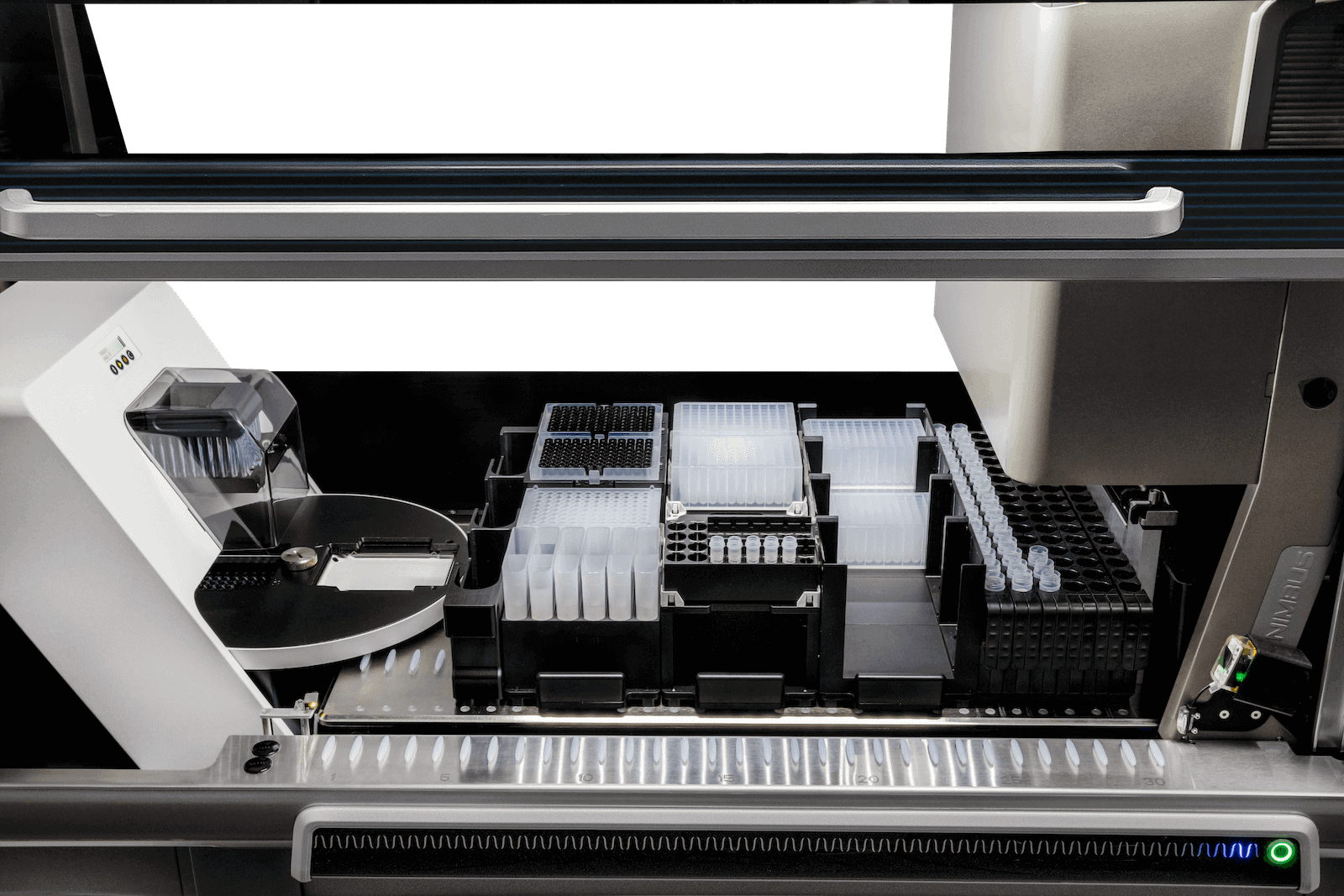
Aligned technology for unmatched capability
Combining advanced liquid handling robotics and proven magnetic particle-based technology, the MT-Prep™ XL delivers reliable high-throughput, high-purity nucleic acid extraction.

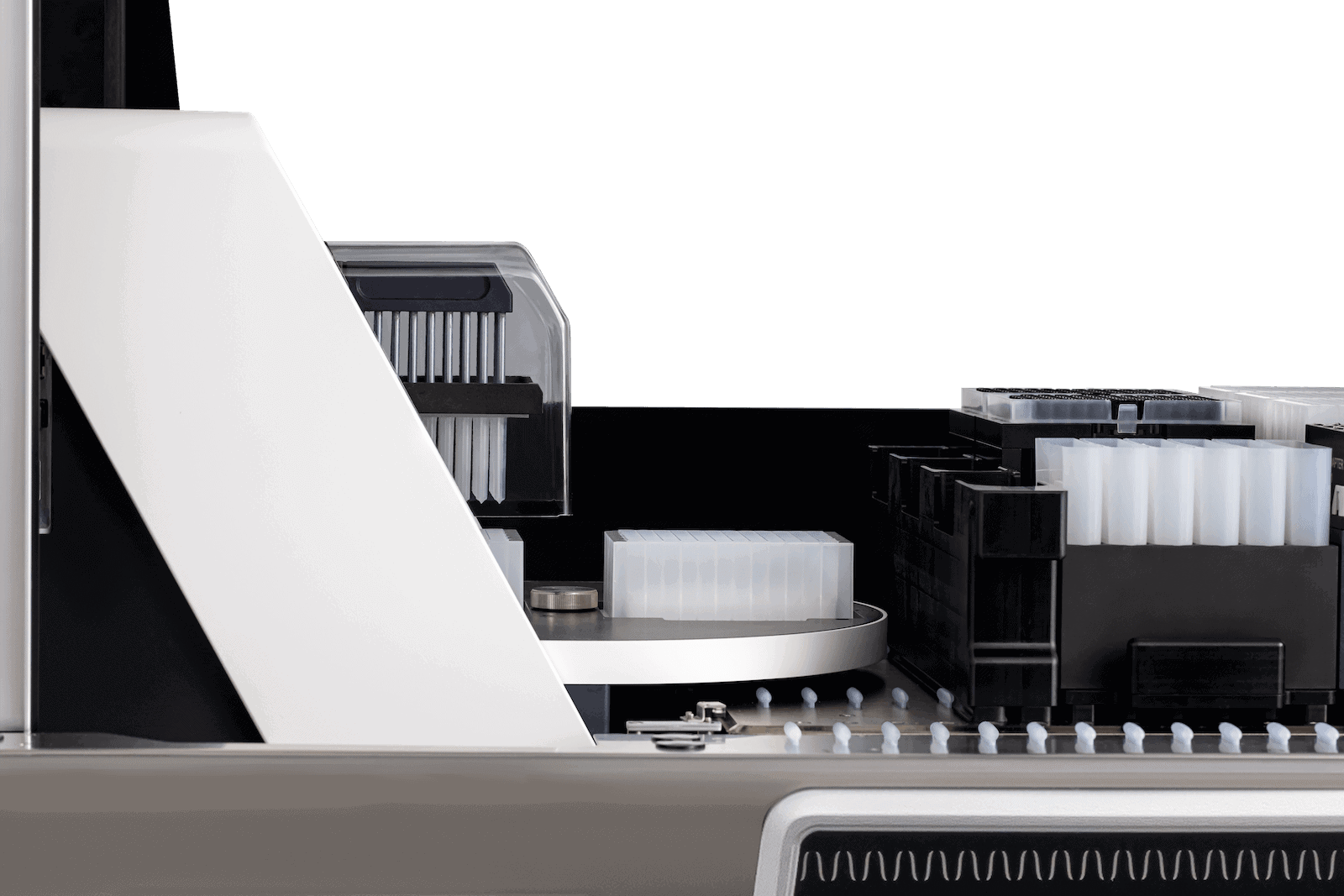
Persistent precision
CO-RE® (Compressed O-Ring Expansion) technology enables maximum accuracy, precision and reproducibility. This is achieved by eliminating tip distortion and aerosol generation during automated pipetting.
Efficient parallel processing
A dual-position turntable allows simultaneous processing of one plate with the magnetic head while another plate is prepared and transported in readiness for subsequent processing. This parallel processing capability results in optimal throughput.
Virtual Demo
Explore the MT-Prep™ XL
System Specifications
130 H cm with door open
CO-RE is a trademark of Hamilton Company. MT-Prep and Puryx are trademarks of AusDiagnostics Pty Ltd.
The MT-Prep™ XL instrument is intended for in vitro diagnostics use to extract nucleic acids from clinical samples by authorised clinical testing laboratories in Australia, and the European Economic Area and is included on the Australian Register of Therapeutic Goods (ARTG), and compliant with the European IVD Directive 98 /79/EC.

