In this edition
- A look at our parent company, R‑Biopharm
- The Award-Winning HighPlex Ascend
- Improvements to our Respiratory panels
- Solutions for vector-borne pathogens testing
- Mpox global health emergency
- Workflow Comparison: Extraction-free PCR, or parallel extraction?
- Company Culture Highlights
- Join a network of diagnostics professionals
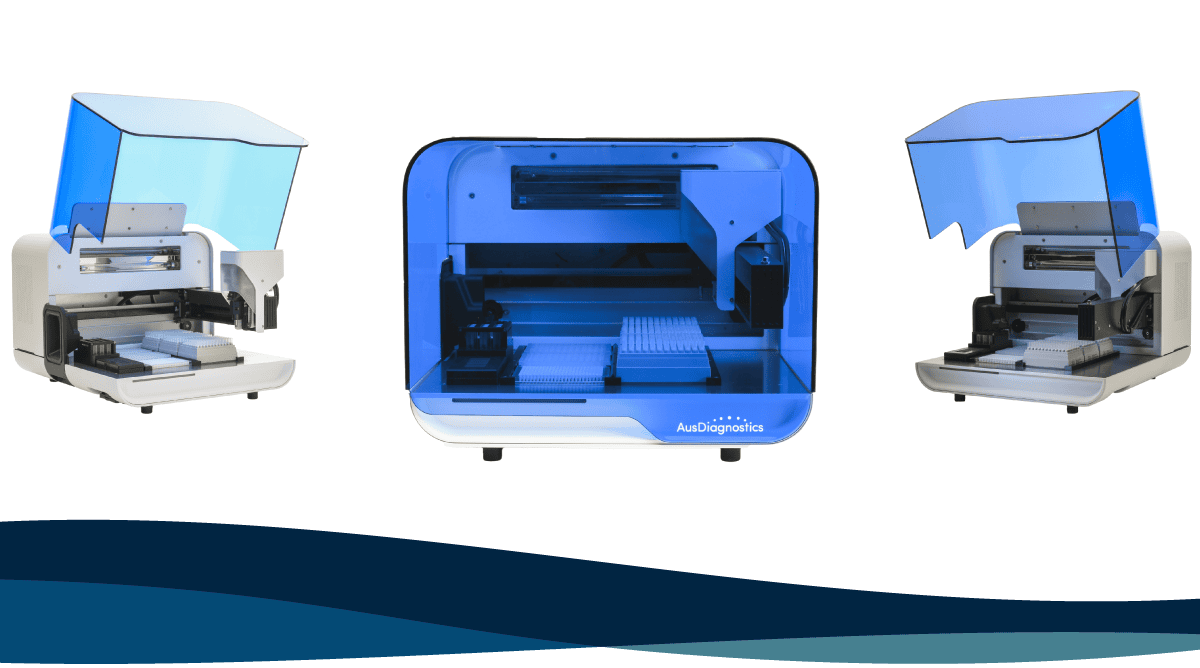
The Award-Winning HighPlex Ascend(Coming Soon) Quality is not merely a standard, but a continuously evolving process. It is the motivation for innovative ideas and all the efforts needed to bring them to life. Last quarter, we announced the HighPlex Ascend; The next iteration of our most popular MT-PCR automation platform. Trusted by laboratories as an everyday diagnostics workhorse, the classic HighPlex is synonymous with quality and reliability. The HighPlex Ascend* was the culmination of decades of experience and a multidisciplinary collaboration of design and diagnostics experts, sharing a singular vision of quality automation.
The effect of these innovations has now been recognised. We are thrilled to share that the HighPlex Ascend* is a Good Design Award winner in the categories of Engineering and Product Design.
The award, presented by Good Design Australia, was justified with reference to the 95% reduction in the number of parts comprising the HighPlex Ascend* compared to its predecessor, improving reliability of quality assurance passes. The lower part count is one factor contributing to a 163% efficiency improvement for assembly, calibration, and commissioning. Additionally, the new design features a range of usability improvements, such as visibility of the instrument deck and status lights, improved component accessibility, and a simple, easy-open instrument canopy. The award is a testament to the AusDiagnostics team’s commitment to continuous improvement, and their wealth of skill and expertise.
* The HighPlex Ascend is coming soon. |
Improvements to our Respiratory panelsWe are committed to continuously improving our products and services based on user feedback. As a result of careful post-market surveillance, we are thrilled to share several upcoming improvements to our range of Respiratory TandemPlex® panels. The product changes were communicated to existing customers in a detailed advisory notice, and will improve the specificity, genotype inclusivity, non‑specific background amplification, robustness, and stability of certain assays. Seemingly every newsletter we publish includes an extensive list of product updates. Behind each list lies an enormous effort by our Research & Development, Quality Assurance, and Product Management teams to understand the plight of our customers and translate their challenges into tangible improvements. We would like to thank our customers for your continued support and collaboration in improving our panels and uplifting patient outcomes.
|

Solutions for vector-borne pathogens testingVector-borne tropical diseases may be caused by a range of viral, bacterial, or protozoan pathogens. The number of candidates and the required range of diagnostic sensitivity poses a challenge for clinicians. We recently launched our Vector-Borne Disease portfolio, a meaningful step toward supporting the response to neglected tropical diseases. The pair of TandemPlex® panels enable clinicians to perform differential diagnostics of syndromically-relevant pathogens spread by mosquitos and livestock.
* The Vector-Borne Disease panels are intended for research use only. Not for use in diagnostic procedures. |
Mpox global health emergencyThe AusDiagnostics team has been closely monitoring the spread of mpox as the World Health Organization (WHO) declared a public health emergency of international concern (PHEIC) in response to a worrying increase in transmission. Monkeypox virus is a lesion-causing virus that readily transmits via skin-to-skin contact. Those with underlying health conditions, especially immunocompromised people, are more likely to experience a higher severity of infection and greater risk of fatality. WHO cited a novel subclade of Monkeypox to justify an internationally coordinated effort to quell the outbreak. The new subclade, designated Ib, is expected to exhibit a high likelihood of infection for close contacts of confirmed cases according to the European Centre for Disease Prevention and Control (ECDC). As health authorities in Europe, UK, USA, and Australia prepare for the arrival of mpox, consider integrating multiplexed syndromic screening into your service offering with the AusDiagnostics Lesions and Ulcers 12-well* panel.
* The Lesions and Ulcers 12-well (REF 87191) panel is intended for research use only. Not for use in diagnostic procedures.
|

Workflow Comparison: Extraction-free PCR, or parallel extraction?As laboratories contend with the unending struggle to optimise their diagnostic workflow, it pays to challenge long-standing conventions and consider a range of alternatives.
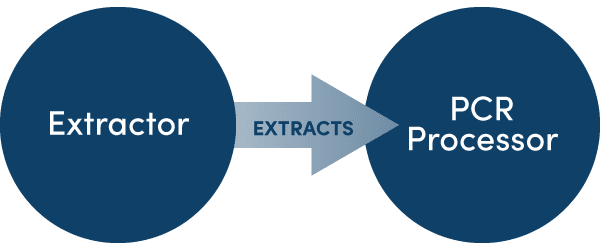
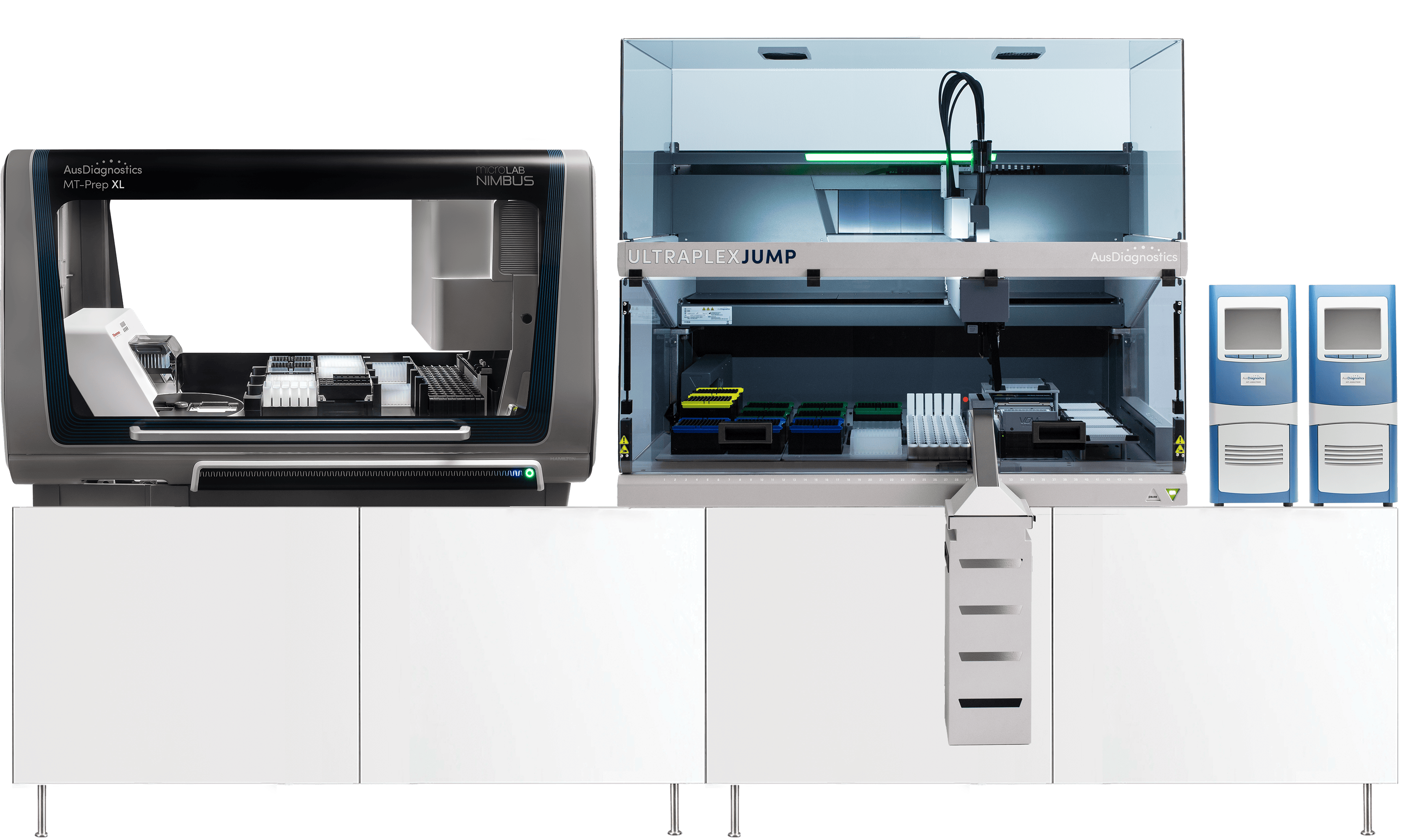
Parallel ProcessingUsers of AusDiagnostics instruments will be familiar with parallel processing. Primary samples are loaded onto an extractor for purification, before being transferred to a PCR processor for amplification and analysis.
Parallel processing has the advantage of simultaneous utilisation of multiple specialised instruments. When all the instruments in a parallel processing workflow are aligned, runtime is generally optimised and operational simultaneity reduces instrument downtime, creating a powerful improvement in throughput over the course of a workday.
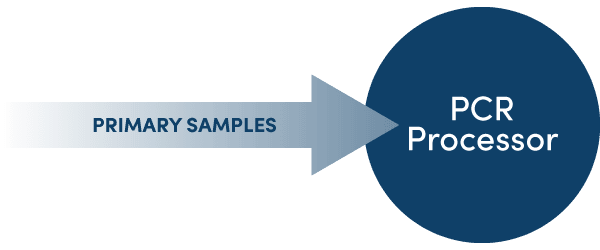
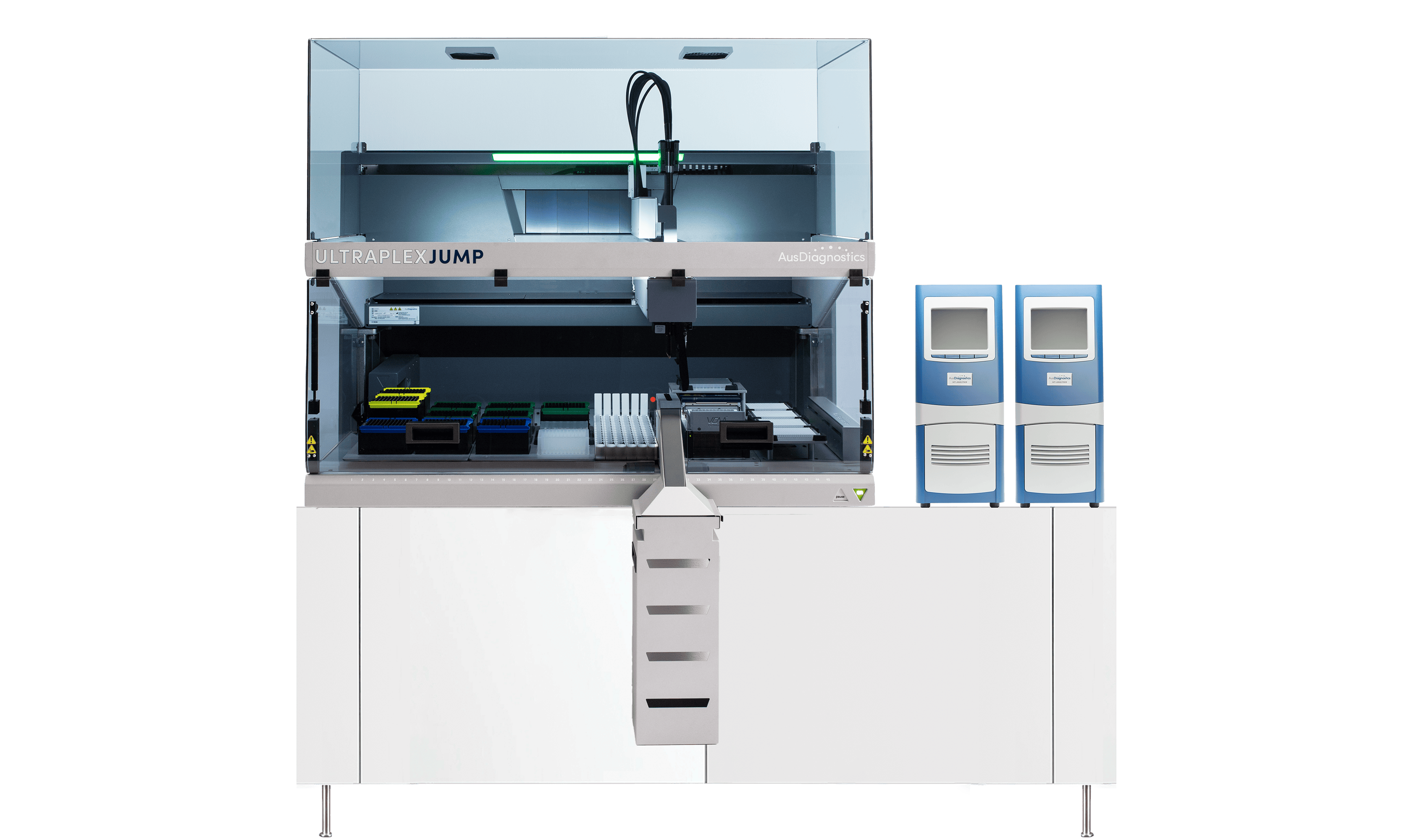
Versatile ProcessingTactical switching between the parallel and extraction-free configurations ensures your laboratory is always able to leverage the optimal workflow for a given situation. Adapting your PCR processor from parallel to an extraction-free configuration can free your extractor for other important processes in the lab, and allows for more rapid screening of targets compatible with pre-treatment protocols. Alternatively, changing to parallel processing ensures maximum throughput when testing for a range of different targets simultaneously. The UltraPlex Jump* (RUO, Coming soon) enables diagnosticians to tactically swap between parallel and extraction-free capabilities for compatible targets, conferring the versatility to select the best workflow for your laboratory’s evolving demands.
* This product is intended for research use only. Not for use in diagnostic procedures.
|

Company Culture HighlightsSeptember was marked by our much-anticipated annual Corporate Games. This year, the team unleashed their competitive spirit with a dodgeball tournament.
The team also took a morning to recognise RUOK day, sharing important mental health check-in conversations over a barbecue brunch.
As part of a shift toward charitable activities, our New Zealand team spent their team-bonding day with Glen Innes, a decile 1 low-socioeconomic school. The team brought some poems, cards, and books, and spoke with staff to better understand the plight of students learning in underprivileged areas. The New Zealand team received several thank you cards from the students.
|

Join a network of diagnostics professionalsOur social media community is a unique blend of professionals from clinical and non-clinical backgrounds. Following AusDiagnostics on social media is the perfect way to connect with an up-to-date network of peers to discuss the latest updates in the diagnostics world.
In case you missed it, here is a taste of our recent updates from Linkedin.
|
| Be the first to receive AusDiagnostics updates: |
Join the conversation:
    ↑ Back to top |